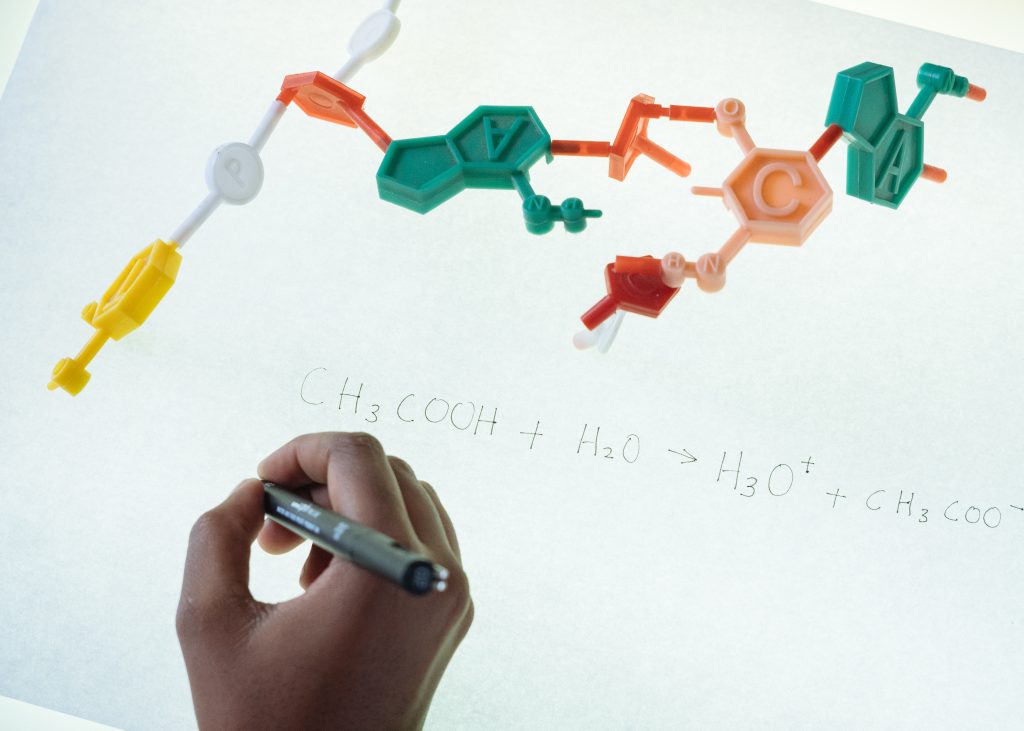
Isomerism: Definition and Significance
Definition: Isomerism refers to the existence of different compounds with the same molecular formula but distinct arrangements of atoms.
Significance: Understanding isomerism is essential for comprehending the intricacies of chemical structures and their corresponding properties.
Types of Isomerism
Structural Isomerism: Molecules with the same molecular formula but different connectivity of atoms, leading to unique physical and chemical properties.
Stereoisomerism: Molecules with the same connectivity of atoms but different spatial arrangements, resulting in differences in optical and geometric properties.
Geometric Isomerism: A subset of stereoisomerism involving compounds with restricted rotation, leading to cis-trans or E-Z isomers.

Importance of Isomerism
Drug Design: Isomerism impacts drug effectiveness and side effects, emphasizing the need for specific isomeric structures.
Materials Science: Distinct isomeric forms contribute diverse properties in materials like polymers and crystals.
Food and Fragrance Industry: Isomerism influences the aromas and flavours of compounds, contributing to diverse culinary experiences.
Also Read –>> Thermal Energy
Isomer Identification
Spectroscopy: Nuclear Magnetic Resonance (NMR) and Infrared (IR) spectroscopy aid in identifying isomeric structures.
Mass Spectrometry: Mass spectrometry helps differentiate isomers based on their mass-to-charge ratios.

Conclusion
Isomerism’s manifestation in molecules unveils the fascinating intricacies of molecular structures. The diversity and significance of isomers reach far and wide, shaping industries, scientific exploration, and our understanding of the molecular world.
Embark on a journey of academic growth with Bright Culture based Novena, Singapore. Our chemistry tuition programs are designed to help you succeed in H2 chemistry, Secondary 1, and 2 Science, and Upper Sec Chemistry. Our experienced chemistry tutors will guide you through the intricacies of chemistry, providing a comprehensive and effective learning experience.